the drug product or service has now been made and packaged. Through an aseptic fill complete process, packaging components and the drug item are sterilized previous to staying combined underneath sterile situations.
A. PUPSIT is a expression Employed in aseptic processing operations, and it stands for pre-use put up-sterilization integrity testing. This tests is performed within the sterilizing filter soon after it truly is mounted ahead of product or service manufacturing and on the other hand after the merchandise manufacturing is completed.
Yet, filter distributors are becoming significantly focused on optimizing pre-filtration processes in order that these programs satisfy the specialised requirements of their supposed software.
The significance of liquid bottle filling equipment lies of their capacity to streamline the complicated process of filling liquid remedies into bottles with unparalleled performance. Classic guide filling strategies had been vulnerable to human problems, resulting in inconsistent dosages, contamination dangers, and increased production time.
Right after Visible inspection on the sealing, use scissors to chop with the guideline over the sealed tubing. Accomplished!
For the risk affiliated with examining the calibration standing of filling device scales ahead of the commence, the influence of lacking this sort of checks without any trusted records has adverse outcomes on Over-all products high quality, for instance products crammed volume. Solution quantity is without doubt one of the essential excellent parameters which should be underneath good Handle. The entire RPN is calculated to be 84 (yellow). Below the workforce uncovered that not using a checklist to report results and also to be added on the BMR may perhaps bring about lack of Handle on these significant manufacturing action.
Making certain there are no current microbes in the atmosphere which can influence the integrity of here goods ahead of ultimate sealing and packaging through environmental monitoring and concluded products screening.
BevSource has the know-how to usher your beverage from smaller-scale beverage creation into a shelf-Completely ready merchandise. Pick us as your pilot generation facility to find out success in the First run and garner the assistance you'll want to get your next actions.
Throughout this process, staff members ended up picked to ensure that the staff consisted of people who possessed the know-how to list the final points for being thought of in the doc.
The world supervisor with QA senior personnel reviewed the current process and located that getting a gadget by using a digital camera or simply a mobile having a camera improve the likelihood of getting images either With all the acknowledgment or not of accompanying personnel. Thus, final decision-makers make your mind up to modify/revise The existing SOP and insert new Recommendations and Management stating that no camera, cell, or any device contains a digital camera to accompany workers or guests in the limited space.
Any parenteral solutions and many implantable devices are the commonest candidates for aseptic processing.
Manufacturing delays. Stringent restrictions governing aseptic manufacturing necessarily mean that there will be extended delays must an item be exposed to microbial contamination.
Tracing its origins supplies Perception into how this innovation has evolved to meet stringent sterility prerequisites.
Some threats linked to numerous ways are in the yellow zone (RPN is concerning 35 get more info and 104). Listed here, the workforce’s determination may differ among accepting it, and further reduction is needed just as much as possible. This discussion has chosen all risks while in the pink zone, as shown in (Table 3). The staff agreed having proper actions with the elimination or mitigation of the risk.
 Andrea Barber Then & Now!
Andrea Barber Then & Now! Kenan Thompson Then & Now!
Kenan Thompson Then & Now!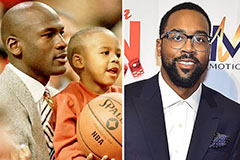 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!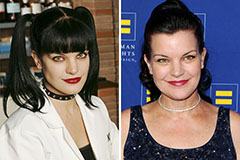 Pauley Perrette Then & Now!
Pauley Perrette Then & Now! Ryan Phillippe Then & Now!
Ryan Phillippe Then & Now!